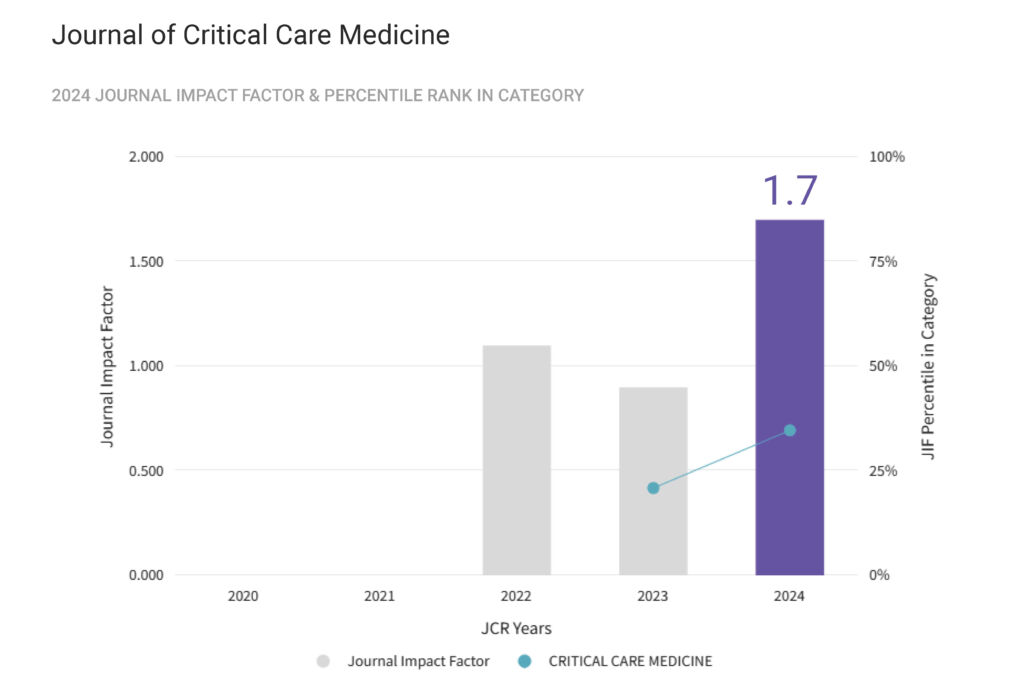
1. Aims and scope
The Journal of Critical Care Medicine (JCCM) is an official Journal of the George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Targu Mures, Romania, and is published quarterly.
JCCM is an international journal dedicated to publishing high-quality peer-reviewed articles about critical care medicine from all around the world. The prominent themes covered by the journal include pathologies related to critical care patients, within a broad spectrum of disciplines and therapeutic areas, including, but not restricted to general surgery, perioperative care, cardiology, internal medicine, pneumology, infectious diseases, organ transplantation, emergency medicine, pediatrics and neurology. Our journal’s scope is to provide for all intensivists the newest information related to the critical ill patients, treatments and their outcomes.
The Journal addresses the entire community of specialists involved in treating critically ill patients, with a particular focus on those from Central and Eastern European countries, aiming to provide studies in critical care medicine with a new regional perspective, specific for this part of Europe, but not restricted to it. The Journal will also publish international, overseas articles, especially those which contribute to a better understanding of pathologies leading to critical illnesses, and which reflect international models of care for these conditions.
2. Editorial process and peer-review
Submitted manuscripts are first checked to ensure that they comply with instructions to authors and are in accordance with the “Uniform Requirements for Manuscripts Submitted to Biomedical Journals” IJCME, and that all references, figures and tables meet the journal’s requirements.
All manuscripts sent to the journal are routinely screened using specialized anti-plagiarism software. In all cases where any possible irregularity exists, the editorial office will follow the principles stated in COPE (Committee on publication ethics) guidelines.
Only manuscripts complying with the above requirements and free of possible irregularities, will be entered into the review process. The author(s) will be informed that the manuscript has been accepted for review.
Authors are invited to suggest the names of potential reviewers and the Editor may choose, without obligation or explanation, to use one or more of these. Authors may also specify the names of a person(s) which they do not wish to review their manuscript, in which case a brief explanation should be given.
All articles will be reviewed by at least two colleagues with expertise in the manuscript’s subject matter. The peer review process is double-blinded. Thus, the identities of reviewers, chosen by the editor, will not be disclosed to authors. Also, the reviewers will not be aware of the author(s) identity. The reviewing process does not involve any costs. The reviewing time is 14 days for new submissions, and 5 days for revised submissions. If the due time is passed, the reviewers will be notified several times. If the reviewers fail to reply, the Editor can unassign the reviewer and select a new one.
The average time from submission to a decision following the first review is approximately 1-3 months.
Based on the reviewers’ opinion, the Editor will choose one of the following alternatives:
- Accepted
- Minor revisions required
- Major revisions required
- Rejected
In cases where revision is required, the author(s) will be invited to amend their manuscript which should be resubmitted as soon as possible, but not later than 6 weeks. The revised manuscript will be reappraised by the initial reviewers and notification of a final decision will be sent to the author in approximately three weeks. If accepted for publication, the science methodology editor will perform a final appraisal of the manuscript before initiation of publication stage (publication fee payment, proofreading).
After acceptance and prior to publication, authors will receive a PDF file with the edited version of their manuscript for final proofreading and will be asked to carefully check the completeness and accuracy of the text, tables and figures. Accepted articles will receive a DOI code and will be published ahead of print immediately after acceptance.
Corrections, retractions, complaints
Our policy is to consider corrections and to publish them only if the author provides compelling evidence that a major claim of the original paper was incorrect (name typing errors, affiliations, wrong numerical data, etc.), or information was disclosed. Corrections that affect the methodology, data analysis, statistical results, conclusions, are not allowed. The corrections will be published as Erratum, in the next issue.
Infringements of professional ethical codes, such as multiple submission, fake authorship, plagiarism, fraudulent use of data will trigger a retraction procedure. A retraction note signed by the authors and/or the editor will be published in the next issue. The original article will not be removed, but marked as Retracted.
Complaints, disagreements over interpretation should be addressed to the editor of the journal or addressed in a letter-to-editor.
3. Submission guidelines
General policies
All manuscripts submitted to JCCM must be original, high quality, and conform to the “Uniform Requirements for Manuscript Submitted to Biomedical Journals” published in Annals of Internal Medicine (1997;126:36-47).
Authors should not submit the same manuscript simultaneously to more than one journal, in the same or different language. The journal does not have article processing charges nor article submission charges, but there is a publication fee following acceptance.
All manuscripts should be submitted through the journal’s editorial manager. The corresponding author is responsible for formatting and uploading all documents. We encourage the use of open-access instrument ORCID ID for authors, which is also used by reviewers, and editorial board members. New accounts can be created manually or by retrieve the details from the ORCID registry.
JCCM complies with CC BY 4.0 license, subscribes to the principles of Committee on Publication Ethics (COPE), and to the International Committee of Medical Journals Editors (ICMJE) recommendations, to review best practice and ethical standards in the conduct and reporting of research and other published materials.
We encourage authors to use the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) Network research reporting guidelines, to improve the reliability and value of the publication: https://www.goodreports.org/ .
Authorship
All individuals listed as authors should qualify for authorship and should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. All individuals listed as authors should qualify for authorship and should have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Authors included in the manuscript should meet all of the following conditions as stated in the ICMJE guidelines:
- Substantial contributions to the conception and design of the work, acquisition, analysis, or interpretation of data;
- Drafting the article or revising it critically, for important intellectual content;
- Final approval of the version to be published.
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Any other contributors, who do not qualify for authorship, should be acknowledged in an acknowledgment section. For further information about authorship, please refer to the ICMJE guidelines.
Authors’ contributions – CRediT taxonomy
JCCM uses the CRediT Taxonomy to define author contributions. Each author on a paper may have one or more CRediT contribution roles. Author contributions will be included at the end of the manuscript. Having a role described by the taxonomy does not automatically qualify someone as an author.
| Contributor role | Role definition |
| Conceptualization | Ideas; formulation or evolution of overarching research goals and aims. |
| Methodology | Development or design of methodology; creation of models |
| Software | Programming, software development; designing computer programs; implementation of the computer code and supporting algorithms; testing of existing code components. |
| Validation | Verification, whether as a part of the activity or separate, of the overall replication/reproducibility of results/experiments and other research outputs. |
| Formal analysis | Application of statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data. |
| Investigation | Conducting a research and investigation process, specifically performing the experiments, or data/evidence collection. |
| Resources | Provision of study materials, reagents, materials, patients, laboratory samples, animals, instrumentation, computing resources, or other analysis tools. |
| Data Curation | Management activities to annotate (produce metadata), scrub data and maintain research data (including software code, where it is necessary for interpreting the data itself) for initial use and later reuse. |
| Writing – original draft preparation | Creation and/or presentation of the published work, specifically writing the initial draft (including substantive translation). |
| Writing – review and editing | Preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary or revision – including pre- or post-publication stages. |
| Visualization | Preparation, creation and/or presentation of the published work, specifically visualization/data presentation. |
| Supervision | Oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team. |
| Project administration | Management and coordination responsibility for the research activity planning and execution. |
| Funding acquisition | Acquisition of the financial support for the project leading to this publication. |
Policy on the Use of Artificial Intelligence (AI) Tools in Manuscript Preparation
JCCM recognizes the growing role of Artificial Intelligence (AI) tools in academic writing. While these technologies may assist in language refinement or idea generation, the integrity, originality, and accountability of scholarly work must be preserved.
This policy is consistent with guidance from:
- The Committee on Publication Ethics (COPE)
- The International Committee of Medical Journal Editors (ICMJE)
- The World Association of Medical Editors (WAME)
AI tools cannot be listed as authors. Only individuals who meet authorship criteria, as defined by the ICMJE guidelines, can be credited as authors. Human authors remain fully responsible for the content, accuracy, and integrity of any work submitted to the journal, and must ensure that AI is used in a limited and ethically sound manner.
If AI tools have been used in the preparation of a manuscript, their use must be transparently declared in the Acknowledgments or Methods, stating:
- The name and version of the tool
- The purpose of its use (e.g., grammar correction, idea structuring)
- A statement confirming that all AI-generated content has been verified by the authors.
4. Types of papers
The Journal of Critical Care Medicine publishes the following types of papers:
a. Reviews
The journal publishes comprehensive review papers on actual topics of interest related to critical care medicine. Review articles should include a brief non-structured abstract of no more than 300 words and the text should be limited to 5.000 words including references, tables, and figures.
Review articles can be submitted by invitation or unsolicited. In both cases, full consideration will be given to articles providing a substantial contribution to a better understanding of a pathophysiological or clinical aspect in a field related to critical care medicine.
b. Research papers
The journal publishes research papers on actual topics of interest related to critical care medicine. These should include a structured abstract of no more than 300 words. The full manuscript should not exceed 5.000 words including references, figures, and tables, being divided into sections headed Introduction, Materials and methods, Results, Discussions, Conclusions.
c. Case reports and case series
Case reports should be limited to the presentation of a single particular and uncommon case or uncommon presentation of a disease. Case series include a description of a series of a maximum of 10 cases with common particularities. The abstract should be limited to 200 words, being divided into introduction, case presentation/presentation of case series, and conclusions. The full manuscript should not exceed 2.000 words including references, figures, and tables, being divided into sections headed Introduction, Case presentation/presentation of case series, Discussions, Conclusions. In manuscripts pertaining to case presentation or case series, the number of authors should be limited to four and the number of references to twenty, and the number of figures to 5.
d. Brief reports
Brief reports refer to articles presenting a short communication related to an original preclinical or clinical study which is not a case presentation or a case series report. While the structure of the abstract and of the full text should be similar to that detailed for full original articles, the length of the manuscript should be shorter, the abstract limited to 200 words, and the full text (including references, tables, and figures) to 2.000 words.
e. Letter to editor
A letter to the editor may refer to an article recently published by the journal, commenting on the article in a constructive professional manner the content of which, in the opinion of the author(s) would add the current status of knowledge in the field. If accepted, the letter will be sent to the authors of the original article who will have the opportunity to respond and to have their response published in the same journal issue as the letter to the editor. The letters should be limited to 500 words, 5 references, and 3 authors. No abstract is required.
f. Editorial
Editorials should be limited to 2000 words (including references) and should be related to an article published in the current number or to a specific topic that is current and of high interest to the readers.
g. State-of-the-art papers
The journal publishes state-of-the-art articles that aim to provide an update on the current status of areas of high interest to critical care medical specialists. The principal aim of such articles is to offer the specialist and other practitioners a source of continuing education and forum for discussion. A state-of-the-art article should have a full text limited to 4.000 words, in addition to a 200-word unstructured abstract. Sections of the article should be divided using headings relevant to each particular case.
5. Manuscript organization
The submission should include the following attachments:
A. Cover letter
All manuscripts should be submitted together with a cover letter attached as a separate file, stating that:
- the manuscript is original
- no portion of the manuscript is under consideration for publication in any other journal or has been previously published, except as an abstract of fewer than 400 words.
- all authors have read and approved the manuscript and accept responsibility for the full content.
- Authors must state all possible conflicts of interest relating to the manuscript, or, if there are none, this should be stated as “none declared”.
The cover letter should be signed by the corresponding author who should clearly mention in the letter`s text that he/she is empowered by all the authors to sign the cover letter and submit the manuscript on their behalf.
The cover letter may include a list of potential reviewers or persons which the author(s) do not wish as reviewers. A brief statement of reasons of suitability/non-suitability should be given.
B. License to publish
A license to publish statement should be signed by the corresponding author on behalf of all the authors. The standard format of this document is available to be downloaded here.
C. Manuscript
The manuscripts, including all tables and references, must be prepared in Word format. The text should be typed double-spaced with no indent, using “Calibri” font size 11.
Please arrange the contents of your manuscript in the following order:
i. Title – Concise and informative. Titles are often used in information-retrieval systems. Avoid abbreviations and formulae where possible.
No personal information of the authors is allowed within the manuscript, because of the double-peer review process
ii. Abstract – an abstract of no more than 300 words should accompany manuscripts relating to original research, case presentations and review articles. This should be structured using the following headings: Introduction, Aim of the study, Material and Methods, Results, Conclusions. Detailed instructions on abstract preparation according to each manuscript type are given below.
iii. Key words – up to 10 keywords should be supplied by the author(s).
iv. Full text – should be formatted in Microsoft Word, double-spaced, single columned. Use headings and subheadings in all the sections. Original research articles should not exceed 5.000 words including references, tables, table legends and figure legends, and should be divided into the following sections:
a. Introduction
This must be presented in a structured format, covering the following subjects, although actual subheadings should not be included:
-
- succinct statements of the issue in question;
- the essence of existing knowledge and understanding pertinent to the issue (reference);
- the aims and objectives of the research being reported relating the research to dentistry, where not obvious.
b. Materials and methods for original research papers
-
- describe the procedures and analytical techniques.
- only cite references to published methods.
- include at least general composition details and batch numbers for all materials.
- identify names and sources of all commercial products e.g. Voltarol® Emulgel® Gel (Company, Town, Country).
- specify statistical significance test methods.
c. Case presentation/presentation of case series
-
- present the evolution of the case in a chronological order, and emphasize the particularities of the case
- the presented information has to comply with the human rights policy, as it is presented in the Publication ethics section
d. Results
-
- refer to appropriate tables and figures.
- refrain from subjective comments.
- make no reference to previous literature.
- report statistical findings.
e. Discussion
-
- explain and interpret data.
- state implications of the results, relate to composition.
- indicate limitations of findings.
- relate to other relevant research.
f. Conclusion
-
- must NOT repeat Results or Discussion
- must concisely state inference, significance, or consequences
When preparing your manuscript, consider the following rules:
-
- Define abbreviations that are not standard the first time they appear in the text, followed by the abbreviation in brackets. Such abbreviations that are unavoidable in the abstract must be defined at their first mention there. Ensure consistency of abbreviations throughout the article
- All references, tables, and figures should be cited in numerical order.
- Language editing will be available during the editorial process, however, authors whose native language is not English are strongly advised to seek appropriate grammatical assistance when preparing the manuscript. Poorly written manuscripts will be returned for improvement before commencing the editorial process.
v. Acknowledgments – please indicate any source of funding including grants, contracts, or any other form of financial support relating to the study. List here those individuals who provided help during the research (e.g., providing language help, writing assistance or proofreading the article, etc.).
vi. References – Number the references in the order in which they are first cited in the text. References should be indicated as full-size Arabic numerals in square brackets placed before punctuation marks.
vii. Reference style – JCCM uses Vancouver citation style with brackets. List all authors if six or less; otherwise list first three and add “et al”. Please abbreviate titles of periodicals according to Index Medicus, or spelled out in full if not listed in Index Medicus. Use the following formats, paying close attention to the use of punctuation i.e. colon (:), semi-colon (;), comma (,) and full-stops (.).
For journal articles:
-
- Kim J, Fitzgerald JG, Sanders AK, Hofman HG. Long term survival following implantation of drug-eluting stents. J Am Coll Cardiol. 2002;42:652-8.
For articles-in-press:
-
- Hendricks-Ferguson VL, Sawin KJ, Peters V, et al. Novice Nurses’ Experiences with Palliative and End-of-Life Communication. J Pediatr Oncol Nurs. 2015. DOI: 1043454214555196. [Epub ahead of print]
For a chapter in a book:
-
- La Rovere MT, Schwartz PJ: Baroreflex sensitivity. In Opie, L: Drugs for the Heart, Sixth Edition. Philadelphia: WB Saunders. 2006, pp.67-93.
For a Book:
-
- Eisen HN. Immunology: an introduction to molecular and cellular principles of the immune response. 5thed. New York: Harper&Row; 1974. P.406.
viii. Units – follow internationally accepted rules and conventions: use the international system of units (SI). If other units are mentioned, please give their equivalent in SI.
D. Figures
Figures should be prepared separately and sent as additional files, in TIF or PNG format. The figures should be prepared at the standard resolution of 300 dpi. All abbreviations used in a figure should be explained in the figure legend. Figure legends should be concise but explicit, enabling a clear understanding of the illustration. Figures and figure legends should be numbered in Arabic numerals in the order of appearance in the text and should not be embedded within the text. Color figures are preferred. Where a figure(s) is reproduced or adapted from another source, the author must first seek permission from both the author and publisher of the original material. Written evidence of permission for reproduction in both print and electronic formats for worldwide distribution must be forwarded with the manuscript and state “Reproduced with permission from…” or “Adapted with permission from…”.
E. Tables
These must be self-explanatory and should not duplicate the text. Tables should be numbered in Arabic numerals in the order of mention in the text and should not be embedded within the text. Instead, each table should be typed on a separate page at the end of the manuscript. All the abbreviations used in the table should be typed as footnotes immediately below the table. Tables should be created with Word’s Insert Table function in order to be editable. Do not submit tables as image files.
F. Appendices
If there is more than one appendix, they should be identified as A, B, etc. Formulae and equations in appendices should be given separate numbering: Eq. (A.1), Eq. (A.2), etc.; in a subsequent appendix, Eq. (B.1), and so on. Similarly for tables and figures: Table A.1; Fig. A.1, etc.
G. Authors’ contribution
Contributor Roles have to be attributed to each Author of a submission. The roles listed are from the CRedit Taxonomy, a classification standard used to ensure that Authors are credited for their contributions toward published scholarly works.
H. Acknowledgments
Mention if the case.
I. Conflict of interest
The manuscript should contain a statement fully disclosing any conflict of interest related to the manuscript. If there are no conflicts of interest, this should be stated as “none declared”. Material and financial support should also be acknowledged.
J. Funding
Please indicate any source of funding including grants, contracts, or any other form of financial support relating to the study. If not the case, please mention “No external funding was received”.
6. Publication ethics
Informed consent
All research studies involving human subjects must have received approval of the appropriate institutional ethics committee and informed consent must be obtained from all the patients participating in the studies, prior to manuscript submission. The manuscript must contain a statement on the informed consent within the “Material and Methods” section.
In cases where the institutional ethics review committee ruled that approval from them was not required or that the need for informed consent was unnecessary, a statement from the committee to this end should be forwarded to the Editor with the manuscript.
Human and animal rights
Studies involving experimental research on animals or humans must conform to the guiding principles of the Declaration of Helsinki. In case of research involving human subjects, the manuscript must contain a statement within the “Material and Methods” section indicating that the study protocol has been approved by the author(s) institutional ethical committee and that all study participants have given informed consent to participate, or that the ethical committee has waived the need for informed consent. In order to respect patient confidentiality and the right to privacy, identifying information such as patient’s names, images, hospital or hospital record details, should not be included in any published material unless the information is essential for the scientific content. If so, written permission must be obtained from the patient, and this permission should be submitted to the editorial office prior to publication. In the case of animal experiments, both national and the institutional guidelines pertaining to the experimental use of laboratory animals should be rigidly followed.
Conflict of interest
The manuscript should contain a statement fully disclosing any conflict of interest related to the manuscript. If there are no conflicts of interest, this should be stated as “none declared”. Material and financial support should also be acknowledged.
Clinical trials
In case of manuscripts presenting clinical trials, the clinical trial should be registered in a public trials registry at or before the time of first patient enrollment, as a condition for consideration for publication. Trials should be preferably registered in ClinicalTrials.gov, but any registry that is a primary register of the WHO International Clinical Trials Registry Platform (ICTRP) is acceptable, in accordance with the guidelines of the International Committee of Medical Journal Editors.